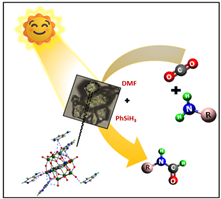
Researchers have discovered a new method of using carbon dioxide under ambient reaction conditions, unlike the harsh thermal conditions reported previously. Using CO2, they have converted amines into N-formamides useful for the synthesis of heterocycles, pharmaceuticals and bioactive compounds through a green approach.
Carbon capture and utilization (CCU) is becoming more and more important as a solution to the increasing greenhouse gases in the atmosphere. Polyoxometalates (POMs), a class of synthetic nanomaterials consisting of three or more transition metals linked together by shared oxygen atoms, are promising candidates for improving the photocatalytic conversion of CO2, the most important greenhouse gas.
They provide high-efficiency catalytic sites and also exhibit exceptional thermal stability, redox potential and semiconductor properties. Using polyoxometalates (POMs) as photocatalysts offers several advantages. Their light absorption properties can be finely tuned by incorporating different transition metals. The secret that makes them promising candidates for photocatalytic conversions is their quick and reversible multielectron transfer properties. However, most of the previous photocatalytic conversions have been carried out under extreme conditions and scientists have been looking for a suitable solution to carry out such conversions under normal conditions.
The use of polyoxometalates (POMs) as photocatalysts aligns with green chemistry principles by reducing the need for stoichiometric reagents and minimizing waste by using CO2 (a greenhouse gas) as a reactant. This contributes to more sustainable chemical processes. Furthermore, POMs as photocatalysts are readily available and cost-effective materials.