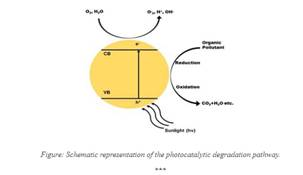
A new metal oxide nanocomposite has been developed that can aid in photocatalytic degradation of organic pollutants such as dyes and pharmaceuticals and hence can be used as sustainable technologies for environmental cleanup.
This metal oxide photocatalysis offers a sustainable solution for the removal of organic pollutants from water bodies. Titanium dioxide (TiO2), zinc oxide (ZnO), and tungsten trioxide (WO3) are remarkable catalysts due to their high surface area and stability. When exposed to light, they generate electron-hole pairs that convert pollutants into harmless by-products. Factors affecting efficiency include the choice of metallic oxide, crystal structure, light parameters, pollutant concentration, pH, and catalyst loading. Optimization of these factors is important for maximizing degradation rates.
Dr. Arundhati Devi and her team at Institute of Advanced Study in Science and Technology (IASST), an autonomous institute of the Department of Science and Technology (DST), have developed a novel metal oxide nanocomposite for photocatalytic degradation of organic pollutants. Fuller’s earth (NiTf) nickel-coated (Ni-doped) titanium dioxide (TiO2) was characterized and tested as a photocatalyst for decolorization of methylene blue. It achieved 96.15% decolorization level of dye solution at pH 9.0 under visible light for 90 min. Fuller’s earth improved the absorption of titanium dioxide (TiO2) in the dark, suggesting a cost-effective environmental photocatalyst. The research work was recently published in the journal Elsevier (Inorganic Chemistry Communications).
The nanocomposites thus constructed may have potential applications in catalysis, energy storage, sensing, optoelectronics (the study and application of electronic devices and systems that detect, sense and control light) through water splitting, as well as in the biomedical field, coatings and renewable energy generation.
Publication link, DOI: https://doi.org/10.1016/j.inoche.2023.110550